H-terminated polycrystalline boron doped diamond electrode for geochemical sensing into underground components of nuclear repositories
Résumé
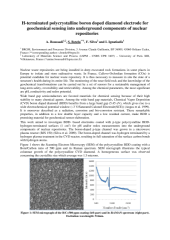
Nuclear waste repositories are being installed in deep excavated rock formations in some places in Europe to isolate and store radioactive waste. In France, Callovo-Oxfordian formation (COx) is potential candidate for nuclear waste repository. It is thus necessary to measure in situ the state of a structure's health during its entire life. The monitoring of the near-field rock and the knowledge of the geochemical transformations can be carried out by a set of sensors for a sustainable management of long-term safety, reversibility and retrievability. Among the chemical parameters, the most significant are pH, conductivity and redox potential. Wide band gap semiconductors are favored materials for chemical sensing because of their high stability to many chemical agents. Among the wide band gap materials, Chemical Vapor Deposition (CVD) boron doped diamond (BDD) benefits from a large band gap (5.45 eV), which gives rise to a wide electrochemical potential window (~3 V/Saturated Calomel Electrode(SCE)) (Angus et al. 1999). It is moreover described as a radiation, corrosion and bio-corrosion resistant. These remarkable properties, in addition to a low double layer capacity and a low residual current, make BDD a promising material for geochemical sensor elaboration. This work aimed to investigate BDD- based electrodes coated with p-type polycrystalline BDD- hydrogen-terminated surfaces (1 cm2) for pH and/or redox measurements into the underground components of nuclear repositories. The boron-doped p-type channel was grown in a microwave plasma reactor (BJS 150) (Silva et al. 2009). The boron-doped channel was hydrogen terminated by a hydrogen plasma treatment in the CVD reactor, resulting in full saturation of the surface carbon bonds with hydrogen atoms. Figure 1 shows the Scanning Electron Microscopy (SEM) of the polycrystalline BDD coating with a Bore/Carbon ratio of 500 ppm and its Raman spectrum. SEM micrograph illustrates the typical columnar growth of the polycrystalline CVD diamond. A homogeneous surface was observed concerning the crystallite size which average was 1.5 microns. On the Raman spectrum of a single crystal diamond intrinsic film (undoped), the diamond peak is usually observed at 1332 cm-1. In Figure 1, the intense peak at 1327 cm-1 corresponding to diamond is shifted due to the "Fano" effect according to doping, which is observed through a broad peak at 910 cm-1. Its intensity shows that the investigated sample was highly doped. Gheeraert et al. (1993) suggested that the peaks at 500 and 1230 cm-1 appears when the boron concentration reaches the critical value of 3×1020 at.cm-3 corresponding to a metallic conductivity. The lack of peak around 1350 cm-1 and 1570 cm-1, which corresponds respectively to D and G graphite peak of impurity phases of non-diamond carbon (sp2), attests to the crystalline quality of the deposit. The slight width at half maximum of the characteristic peak of diamond compared to that of natural diamond reflects the degree of organization and structural perfection of this phase indicating that the coating was of high quality. Electrodes made in this way have been used for 8 month without any surface treatments or conditioning. The electrochemical behavior of Hydrogen-terminated BDD was studied by cyclic voltammetry. Electrodes showed a wide potential range of about 2 V/SCE. They also showed and a rapid reversible charge transfer in the presence of redox probes such FeCN63-/4- and Ru(NH)63+/2+. Performances, reliability and robustness for pH or redox monitoring were examined by potentiometric measurements at 25°C under anaerobic conditions (oxygen-free atmosphere, 100 % nitrogen) in a glove box. Investigation has been limited in pH, ranging from 5.5 to 13.5, close to those encountered in the environment of the nuclear repositories. The feasibility of measuring pH with BDD electrodes was first tested in NH4Cl/NH3-NaCl (0.1mol L-1) buffer solutions, leading to electrode calibration over the widest range of pH, from around neutral to basic pH. Experiments were also conducted in NaHCO3/Na2CO3 buffer samples, similar to conditions prevailing in the COx formation. For redox measurements, [Fe3+]/[Fe2+] ratios were analysed at different pH and/or ionic strengths (supporting electrolytes concentration ranged from 0.05 to 1 mol.L-1). The same measurements were also done using a 10-mm disk platinum electrode with a surface of 78.54 mm². No pH sensitivity was observed, thus the energy level of the state was not moved. However, for redox measurements the potential acquired by Hydrogen-terminated BDD and Platinum electrode converged to a value of the same order of magnitude, independently of the sample. This fact demonstrates that, under the same experimental conditions, the redox couples fixe identically the potential of the electrodes. Investigations with reference to ionic strength in thermodynamically equilibrated Fe(III)/Fe(II) samples were highly interesting. Independently of the electrode, the voltage measurement was not or little affected, whereas both the solution conductivity as well as the speciation were affected, due to the increase in salinity. This means that the term [Fe3+]/[Fe2+] is practically unaffected. This implies that assuming the ratio of the activity coefficients, γFe3+/ γFe2+ as equal to 1 has a minor effect on the measured redox potential. H-terminated BDD electrode appears well suited for redox monitoring. Work is in progress to demonstrate the robustness of the H-terminated BDD electrode for redox monitoring into COx over a long period.
Fichier principal
 Betelu_BRGM_abstract_Montpellier_2012_poster_H-terminated_polycrystalline_boron_doped_diamond_electrode_.pdf (164.19 Ko)
Télécharger le fichier
Betelu_BRGM_abstract_Montpellier_2012_poster_H-terminated_polycrystalline_boron_doped_diamond_electrode_.pdf (164.19 Ko)
Télécharger le fichier
Origine : Fichiers produits par l'(les) auteur(s)
Loading...
